Current & Future of Hydrogen Fuel Cells : Technology, Use Cases & future
Hydrogen and fuel cell technologies have significant potential to enable this transition to a clean, low-carbon energy system. Completing this transition will result in greatly reduced greenhouse gas emissions and improved air quality.
In fact, in order to achieve the objectives of the energy transition, scientific all around the world are now interested in the production and use of low-carbon and renewable hydrogen. Used to date mainly in the chemical and refining industries, this energy carrier could contribute to decarbonising certain industrial sectors, ensuring the storage of electricity or powering the transport sector. However, the deployment of hydrogen technologies is still awaiting the removal of a number of obstacles.
Hydrogen can produce heat and power engines by direct combustion: piston engines, gas turbines and rocket engines, in all cases with pure water as the waste product. But above all, in fuel cells it can produce electricity and recoverable heat directly, again with water as the only waste product.
Similar to batteries, a fuel cell is a device that converts energy stored in molecules into electricity through an electrochemical reaction. The operating principle of the hydrogen/oxygen (or hydrogen/air) fuel cell is extremely simple: it involves the oxidation of hydrogen and the reduction of oxygen within a fuel cell leading to the overall chemical reaction:
H_2+1/2 O_2→ H_2 O+We+ ∆Q
with simultaneous production of water, electrical energy We and heat ∆Q.
This reaction takes place in a stack, which is essentially composed of two electrodes – the anode and the cathode – separated by an electrolyte.
At the anode, the electrochemical oxidation of hydrogen
H_2+1/2 O_2→2H^++2e^-
produces 2 H+ protons which pass through the electrolyte and 2 electrons which pass into the external circuit producing the electric current and will electrochemically reduce the oxygen producing water
1/2 O_2+2H^++2e^-→H_2 O
These reactions can take place at room temperature thanks to a catalyst, platinum, which promotes the breaking of chemical bonds in the hydrogen and oxygen molecules.
The overall chemical reaction (H_2+1/2 O_2→ H_2 O) is independent of the nature of the electrolyte and the catalytic nature of the electrode does not intervene in the intermediate electrochemical reactions, but only by increasing their reaction rates, thus the intensity of the electric current.
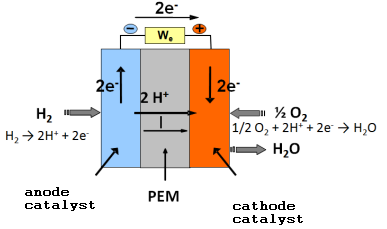
Figure 1: Schematic diagram of a PEM fuel cell.
Scientists and inventors have designed many different types and sizes of fuel cells in the search for greater efficiency, and the technical details of each kind vary. Many of the choices facing fuel cell developers are constrained by the choice of electrolyte. The design of electrodes, for example, and the materials used to make them depend on the electrolyte. Today, the main electrolyte types are alkali, molten carbonate, phosphoric acid, proton exchange membrane (PEM) and solid oxide. The type of fuel also depends on the electrolyte. Some cells need pure hydrogen, and therefore demand extra equipment such as a “reformer” to purify the fuel. Other cells can tolerate some impurities, but might need higher temperatures to run efficiently. Liquid electrolytes circulate in some cells, which requires pumps.
The type of electrolyte also dictates a cell’s operating temperature–”molten” carbonate cells run hot, just as the name implies.
| Type | Electrolyte | Ionic species | Anode catalyst | Cathode catalyst | T(°C) | Area of application |
|---|---|---|---|---|---|---|
| AFC | Potash (liquid) | OH- | Pt | Pt-Ag | 80 | Space, transport, stationary application Range (1 -100 kW) |
| PEMFC & DMFC | Polymer | H+ | Pt(DMFC:Pt/Ru) | Pt | 25-90 | Mobile applications, transport, stationary application Range : 10 mW – 1MW |
| PAFC | Phosphoric acid (liquid) | H+ | Pt | Pt | 180 -200 | Transport, stationary application Range : 100 kW – 1MW |
| MCFC | Molten salts (liquid) | CO3 2- | Ni | Ni-LiO | 650 | Stationary applications Range : 100 kW -10 MW |
| PCFC | Ceramic (solid) | H+ | Perovskite | Pr2NiO4+ | 400-600 | Transport, stationary application Range : 100 W – 10 kW |
| SOFC | Ceramic (solid) | O2- | Ni-YSZ | LaxSr1-xMnO3 | 600 -1000 | Transport, stationary application Range : 100 W – 10 MW |
It should be noted that other types of batteries are being researched but are at the stage of basic laboratory studies and are not covered in this sheet. laboratory studies, they are not mentioned in this fact sheet. For example, MFCs (Microbial Fuel Cell) which produce electricity by digesting microbes in the context of wastewater treatment.
The state of development of the various fuel cell technologies
AFC: Alkaline Fuel Cell – Alkali fuel cells operate on compressed hydrogen and oxygen. They generally use a solution of potassium hydroxide (chemically, KOH) in water as their electrolyte. Efficiency is about 70 percent, and operating temperature is 150 to 200 °C. They require pure hydrogen fuel, however, and their platinum electrode catalysts are expensive. And like any container filled with liquid, they can leak.
MCFC: Molten Carbonate fuel cells – Molten Carbonate fuel cells (MCFC) use high-temperature compounds of salt (like sodium or magnesium) carbonates (chemically, CO3) as the electrolyte. Efficiency ranges from 60 to 80 percent, and operating temperature is about 650 °C. The high temperature limits damage from carbon monoxide “poisoning” of the cell and waste heat can be recycled to make additional electricity. Their nickel electrode-catalysts are inexpensive compared to the platinum used in other cells. But the high temperature also limits the materials and safe uses of MCFCs–they would probably be too hot for home use. Also, carbonate ions from the electrolyte are used up in the reactions, making it necessary to inject carbon dioxide to compensate.
PAFC : Phosphoric Acid fuel cells – Phosphoric Acid fuel cells (PAFC) use phosphoric acid as the electrolyte. Efficiency ranges from 40 to 80 percent, and operating temperature is between 150 to 200 °C. PAFCs tolerate a carbon monoxide concentration of about 1.5 percent, which broadens the choice of fuels they can use. If gasoline is used, the sulfur must be removed. Platinum electrode-catalysts are needed, and internal parts must be able to withstand the corrosive acid.
SOFC: Solid Oxide fuel cells – Solid Oxide fuel cells (SOFC) use a hard, ceramic compound of metal (like calcium or zirconium) oxides (chemically, O2) as electrolyte. Efficiency is about 60 percent, and operating temperatures are about 1000 °C. At such high temperatures a reformer is not required to extract hydrogen from the fuel, and waste heat can be recycled to make additional electricity. However, the high temperature limits applications of SOFC units and they tend to be rather large. While solid electrolytes cannot leak, they can crack.
PEM: Proton Exchange Membrane Fuel cells – Proton Exchange Membrane Fuel cells fuel cells work with a polymer electrolyte in the form of a thin, permeable sheet. Efficiency is about 40 to 50 percent, and operating temperature is about 80 °C. The solid, flexible electrolyte will not leak or crack, and these cells operate at a low enough temperature to make them suitable for homes and cars. But their fuels must be purified, and a platinum catalyst is used on both sides of the membrane, raising costs.
1) The main areas of application of the fuel cell
– Mobile application
In this family we include essentially the mobile phone (which requires a power of the order of
100 mW to a few W), the laptop computer (which requires a power of around 20 W), educational toys and military applications. These sectors are experiencing very strong growth but are increasingly handicapped by the autonomy of their batteries, typically the lithium-ion battery.
Today, the latter has a specific energy of around 130 to 150 Wh/kg, which gives a few dozen hours of autonomy to a top-of-the-range active phone (smartphone type) and around 3 hours for a laptop. However, their users demand 5 to 10 times more.
– Transport
This is the field of application that led to the development of the fuel cell in the early 1990s. With the first tests of PEMFC buses by the Canadian company Ballard, hundreds of prototypes of fuel cell electric vehicles (cars and buses) have been produced since 1993, all of them equipped with PEMFC. They are manufactured by major car manufacturers such as: Daimler, Volkswagen, General Motors, Ford, Toyota, Nissan, Honda, Hyundai and the Chinese SAIC.
The current state of the art is, for each manufacturer, the validation of its technology. Nevertheless, the first commercialisations announced have, for some, as mentioned above, become effective -the end of 2014 for Hyundai (ix35), the end of 2015 for Toyota (Mirai), the end of 2016 for Honda (Clarity), and are expected to become effective for most other manufacturers too.
Also noteworthy is a rapidly growing application (over 12,000), particularly in the US, of fuel cell generators to power electric forklifts.
– Stationary application
With the new laws on deregulation of the electricity sector and trends towards decentralisation of power generation, this sector is beginning to interest many decentralisation of power generation, this sector is beginning to interest many industrialists, particularly in Japan and the USA and recently in Europe.
2) Research and Development Goals
Different laboratories, universities and industry partners are working together to overcome critical technical barriers to fuel cell development. Cost, performance, and durability are still key challenges in the fuel cell industry.
– Cost :
Research, development, and demonstration (RD&D) focuses on the development of low-cost fuel cell stack and balance of plant (BOP) components and advanced high-volume manufacturing approaches to reduce overall system cost. Platinum represents one of the largest cost components of a direct hydrogen fueled polymer electrolyte membrane fuel cell, so there is emphasis on approaches that will increase activity and utilization and reduce the content of current platinum group metal (PGM) and PGM-alloy catalysts, as well as PGM-free catalyst approaches for long-term applications.
– Performance :
To improve fuel cell efficiency and performance, RD&D focuses on innovative materials and integration strategies. Efforts include developing ion-exchange membrane electrolytes with enhanced efficiency and durability at reduced cost; improving membrane electrode assemblies (MEAs) with high power density through integration of state-of-the-art MEA components; modeling to understand system design and operating conditions; and developing stacks with high efficiency at rated power and high-performing BOP components, such as air management components with low parasitic losses.
– Durability :
Fuel cell applications generally require adequate performance to be maintained over long periods of time. In the most demanding applications, system reliability and robustness is required under dynamic and harsh operating conditions. Realistic operating conditions include starting and stopping, freezing and thawing, impurities in the fuel and air, and humidity and dynamic load cycles that result in stresses on the chemical and mechanical stability of the fuel cell system materials and components. RD&D focuses on identifying and understanding the fuel cell degradation mechanisms and developing materials and strategies to mitigate their effects.






Leave a Comment